 |
 |
Introduction Cellular respiration refers to the process of converting chemical energy existing within organic molecules, such as sugars, into a form which can be immediately used by organisms. This process is required for both aerobic (organisms which have oxygen available) and anaerobic (organisms which do not have oxygen available) organisms, but it is executed differently based on the compounds and structures local to the host. However, both processes share the common goal of harvesting biological energy stored in fuel molecules (such as glucose) and convert this energy into ATP. The reaction is written as follows:
ADP and ATP
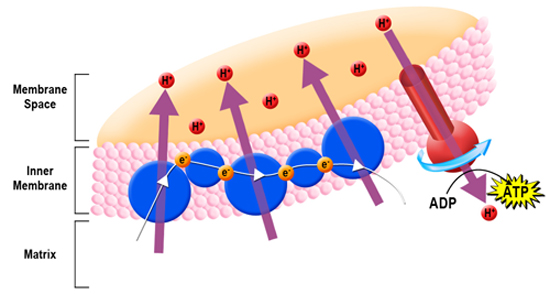
Adenosine triphosphate, or ATP, is often referred to as the energy currency of the cell (Figure 1). It is used in the majority of cellular functions including active transport across a membrane, cell structure maintenance, DNA and RNA synthesis, protein synthesis, etc. However, even though this molecule is extremely important, sometimes more ATP is required than is available. When ATP levels become too low, a special protein signals the cell to begin respiration. Respiration occurs in anaerobic and aerobic organisms, therefore, there is more than one pathway used to produce ATP. However, a high level analysis can conclude that in both types of organisms, insufficient concentrations of ATP can trigger a series of metabolic reactions which ultimately replenish the local ATP supply. These reactions are primarily catabolic; in other words, they break down big molecules into smaller pieces. Free energy is released when the molecules are broken down, which can be sequentially used in each proceeding step. Through a series of oxidation reactions, the cell converts carbohydrates into carbon dioxide and water. It also transforms adenosine diphosphate (ADP) into ATP by adding a free phosphate molecule to ADP. This set of reactions provides a constant source of energy for the cell, as long as all the critical components for the reactions are available. Glycolysis The first stage of respiration is called glycolysis. Glycolysis occurs with or without oxygen and takes place in the cytoplasm. During glycolysis, glucose is broken down to provide energy for the cell. The glycolytic pathway is highly conserved amongst organisms, and is found in all living organisms. Glucose contains high energy bonds which release electrons (energy) when broken. Through this process, the six-carbon glucose molecule is broken down into two, three-carbon molecules called pyruvic acid (pyruvate). Though the bonds holding pyruvate together contain a great deal of potential energy, this step yields little energy. Only two ATP molecules and two nictotinamide adenine dinucleotide (NADH) molecules are produced during glycolysis. Aerobic Respiration vs. Fermentation The next stage of respiration depends on if the organism is aerobic or anaerobic (Figure 3), or in the case of multicellular organisms such as plants, if oxygen is available to the cell. In an aerobic environment, the citric acid cycle begins. Conversely, if an anaerobic environment exists, fermentation or anaerobic respiration takes place. Plants primarily engage in aerobic respiration. However, in rare cases such as extremely wet soil which results in waterlogged roots, anaerobic respiration may also occur in plants. The steps involved in aerobic respiration, anaerobic respiration, and fermentation vary, but all involve the manipulation of electrons and serve to perpetuate the availability of energy for the cell. In aerobic respiration, pyruvate molecules are shuttled from the cytoplasm into the mitochondria to prepare for the citric acid cycle. An enzyme complex called pyruvate dehydrogenase complex produces acetylcoenzyme A (acetyl-CoA) by initially breaking down pyruvate to a two-carbon acetyl group and carbon dioxide. The acetyl group is then transferred to coenzyme A. Coenzyme A then acts as a carrier for the twocarbon acetyl group and transfers the acetyl group to the citric acid cycle. The Citric Acid Cycle The citric acid cycle, also known as the Krebs cycle or tricarboxylic acid cycle (TCA cycle), is an eight step process in which the acetyl group is oxidized to carbon dioxide. The citric acid cycle engages both anabolic and catabolic reactions to construct and break down large molecules. During this process, two important electron shuttle molecules [flavin adenine dinucleotide (FAD) and nicotinamide adenine dinucleotide (NAD+)] are reduced forming FADH2 and NADH. These molecules shuttle electrons to the next step of aerobic respiration, The electron transport chain, ETC, performs the final series of biochemical reactions (Figure 2). Ultimately, the ETC functions to regenerate oxidized molecules (coenzymes) from their reduced state so that other glucose molecules can be converted to energy through future rounds of respiration. To accomplish this objective, electrons (and hydrogen ions) from FADH2 and NADH are transferred to enzyme complexes embedded in the mitochondria’s inner membrane. These complexes act as electron acceptors, passing the electrons from one complex to the next. Since oxygen has a very high affinity for electrons, aerobic respiration is the most efficient means of producing ATP (up to 34 molecules are generated per round).
Fermentation
Eukaryotic and prokaryotic organisms use fermentation in anaerobic conditions. In these cases, the citric acid cycle and the ETC are not used. Instead, pyruvate is the final substrate. These molecules are metabolized in the cytoplasm to produce alcohol or lactic acid, depending on the organism. The main function of fermentation is the reduction of NADH to NAD+. This contributes to the propagation of the glycolytic pathway, which allows ATP production to continue. Recall that glycolysis nets two ATP molecules per glucose molecule. Fermentation allows for the generation of much less ATP per glucose molecule than aerobic respiration because the citric acid cycle and the ETC are not engaged.
Industrial Applications Fermentation has many industrial applications, many of which are evident within the food industry. For example, the sour or tart taste of yogurt and sauerkraut is due to bacteria in the Lactobacillus genus. Lactobacillus perform lactate fermentation in which pyruvic acid (from glycolysis) is reduced directly to the final end product, lactic acid. Another example of industrial fermentation focuses on yeast. Yeast, a unicellular eukaryote, has been used to make leavened (rising) bread for centuries. When yeast undergoes fermentation, carbon dioxide is trapped between gluten and causes the bread to rise. Ethanol is another byproduct of fermentation and is responsible for the alcohol content in beer. Carbon dioxide produced through fermentation is responsible for the effervescence in beer. Respirometers Scientists may use respirometers to measure the rate of respiration. This device capitalizes on the ideal gas law for its function. The ideal gas law applies to those gases in which all collisions between atoms or molecules are perfectly elastic and no intermolecular forces exist. The internal energy is completely kinetic, and any change in energy will be accompanied by a change in temperature, volume, and pressure. The relationship is defined by the ideal gas law equation: PV = nRT In this equation, P is the absolute pressure, V is the volume, R is the universal gas constant (8.3145 J/mol ° K), and T is the absolute temperature (Kelvin). Respirometers typically consist of a device to absorb carbon dioxide produced through respiration and measure oxygen uptake by displacement of fluid in a tube connected to the sealed chamber. Since the carbon dioxide is absorbed, air will be taken in through the tube (displacing the fluid) to maintain equal pressure within the chamber.
Definitions
|
||||||||||||||||||||||||||||||||||||||||||||
| © 2013 eScience Labs, LLC. All Rights Reserved |
 Yeast has been used to make leavened bread for centuries. When yeast undergoes fermentation, carbon dioxide is trapped between gluten and causes the bread to rise. Ethanol, another by product of yeast fermentation, generates the alcohol content in beer, and the
carbon dioxide provides effervescence.
Yeast has been used to make leavened bread for centuries. When yeast undergoes fermentation, carbon dioxide is trapped between gluten and causes the bread to rise. Ethanol, another by product of yeast fermentation, generates the alcohol content in beer, and the
carbon dioxide provides effervescence.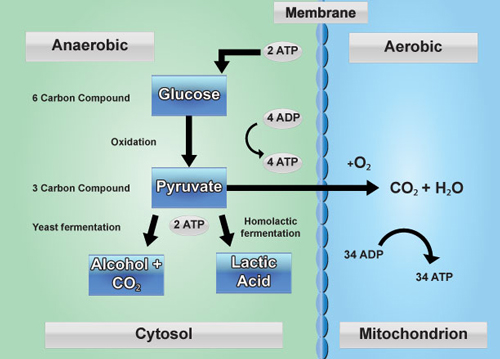
 Cells require more energy during physical activity than when the
body is at rest. Humans rely on aerobic respiration to provide this
energy. However, some physical activities (such as a strenuous
workout) may create an anaerobic environment if the oxygen supply
depletes before the demand is removed. When this occurs, lactate
fermentation will begin to provide additional energy without the use
of oxygen. Lactic acid is a 3-carbon molecule. Therefore, most of
the energy remains and the only energy produced is the two ATP
molecules generated from glycolysis. The buildup of lactic acid can
cause muscles to feel sore and stiff. This feeling persists until the
lactic acid is removed from the muscles by diffusion into the blood.
Cells require more energy during physical activity than when the
body is at rest. Humans rely on aerobic respiration to provide this
energy. However, some physical activities (such as a strenuous
workout) may create an anaerobic environment if the oxygen supply
depletes before the demand is removed. When this occurs, lactate
fermentation will begin to provide additional energy without the use
of oxygen. Lactic acid is a 3-carbon molecule. Therefore, most of
the energy remains and the only energy produced is the two ATP
molecules generated from glycolysis. The buildup of lactic acid can
cause muscles to feel sore and stiff. This feeling persists until the
lactic acid is removed from the muscles by diffusion into the blood.